Japanese scientists achieve 37% success deleting extra chromosome in down syndrome cells using CRISPR

- Japanese researchers removed extra chromosome 21 with CRISPR-Cas9 in lab cells.
- Technique restored normal gene expression and cell functions.
- Early study signals potential path to address Down syndrome root cause.
Over 6,000 children enter the world each year in the United States carrying an extra copy of chromosome 21, a genetic variation that defines Down syndrome and shapes their development from the start.
This condition, known as trisomy 21, arises when cells contain three copies of chromosome 21 instead of the usual two.
It leads to a range of effects, including intellectual disabilities, distinctive facial features, and heightened risks for heart defects, hearing loss, and thyroid issues.
Worldwide, the incidence stands at one in every 1,000 to 1,100 live births, affecting millions across generations. In Brazil alone, records indicate around 300,000 individuals live with the syndrome.

Current approaches to managing Down syndrome focus on supportive therapies. Early interventions such as physical therapy improve motor skills, while speech therapy aids communication.
Medical screenings catch congenital heart disease, present in about half of cases, allowing timely surgeries.
Yet these measures address symptoms rather than the underlying genetic structure. For decades, altering the chromosomal makeup itself remained beyond reach.
Enter CRISPR-Cas9, a gene editing tool that functions like molecular scissors. Developed in the early 2010s, it allows precise cuts in DNA sequences, enabling repairs or removals.
Scientists have applied it to conditions like sickle cell anemia, where faulty genes are corrected. Now, in a novel application, researchers target entire chromosomes.
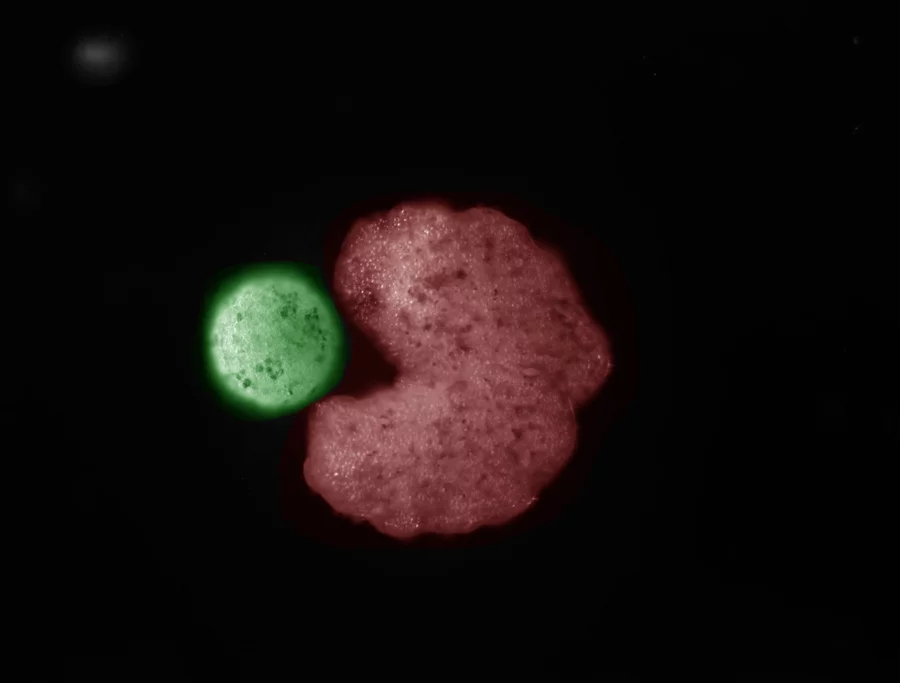
At Mie University in Japan, a team led by Dr. Ryotaro Hashizume pioneered a method to eliminate the surplus chromosome 21.
Their work, detailed in a February 2025 publication, marks the first successful targeted removal in human cells derived from those with Down syndrome.
The approach, termed allele-specific multiple chromosome cleavage, uses CRISPR to make strategic breaks in the extra chromosome, prompting the cell to discard it.

The process begins with cells from affected individuals. Hashizume’s group worked with induced pluripotent stem cells, which can develop into various tissue types, and fibroblasts, mature skin cells that do not divide rapidly.
These choices reflect real-world challenges, as many body cells, including brain neurons, cease division after maturity.
To execute the edit, the team designed guide RNAs to direct Cas9 enzymes toward specific sites on the extra chromosome.
By focusing on allele differences—subtle variations between chromosome copies from each parent—they avoided damaging the two essential ones.
Suppressing the cell’s DNA repair pathways boosted the odds of complete removal, as unrepaired breaks lead to chromosome loss during cell division.
Results showed promise. In treated fibroblasts, the extra chromosome vanished in up to 37.5 percent of cases, restoring the standard pair.
Stem cells responded similarly, with efficiencies reaching 30.6 percent in some lines. Post-editing, cells exhibited normalized behaviors.
Gene expression patterns aligned with those in typical disomic cells, where only two chromosome 21 copies exist.

Proliferation rates quickened, with doubling times shortening by measurable margins.
Antioxidant defenses strengthened, cutting reactive oxygen species levels that otherwise stress mitochondria and accelerate aging.
Genes linked to nervous system growth activated more robustly, while metabolic pathways quieted, hinting at reduced cellular strain.
This restoration matters because Down syndrome disrupts over 200 genes on chromosome 21, including those for amyloid precursor protein, tied to early Alzheimer’s onset in many affected adults.
By 2021 data, global deaths among children and adolescents with the condition totaled 20,810, often from complications like respiratory infections or leukemia.
The study’s reach extends to non-dividing cells, a hurdle in prior gene therapies.
Fibroblasts, which mimic quiescent tissues, responded well, suggesting applicability to brain or heart cells where division halts early.
| Statistic | Value | Source Context |
|---|---|---|
| U.S. prevalence | 1 in 775 births | CDC estimates |
| Annual U.S. births with Down syndrome | About 6,000 | Based on birth rates |
| Global incidence | 1 in 1,000-1,100 live births | United Nations data |
| Study removal efficiency in fibroblasts | Up to 37.5% | Mie University research |
| Efficiency in stem cells | Up to 30.6% | Lab results |
| Global child/adolescent deaths (2021) | 20,810 | Health studies |
Such figures underscore the scale. In countries like Malta, rates climb to 98.9 per 100,000 population, while Brunei follows at 97.2.
Maternal age influences risk, with odds rising from one in 1,500 for women under 25 to one in 100 for those over 40.
Beyond statistics, the technique opens doors to regenerative medicine.
Imagine editing stem cells to produce healthy tissues for transplants, easing heart defects that affect 50 percent of cases.
Or applying it to lab-grown models to test drugs targeting leukemia, which strikes at 10 to 20 times higher rates in this group.
Challenges persist. Off-target effects, where CRISPR cuts unintended sites, demand refinement.
The method’s current form suits cell cultures but requires adaptation for living organisms. Animal models, perhaps mice engineered with trisomy, could test safety next.
Ethical questions arise too. Prenatal applications might spark debates on genetic selection, echoing concerns with embryo editing.
Regulatory bodies like Japan’s Ministry of Health would scrutinize any clinical path, balancing benefits against risks.

Funding streams, from government grants to biotech firms, fuel progress. CRISPR’s patent landscape, involving institutions like the Broad Institute, influences commercialization.
As Hashizume’s team refines guide molecules for precision, collaborations expand.
International groups explore similar tactics for other trisomies, like Edwards syndrome (trisomy 18), which claims most infants within months.
What if this extends to polyploidy in cancers, where extra chromosomes drive tumors? The parallels intrigue oncologists.
Ongoing trials in related fields, such as CRISPR for muscular dystrophy, provide blueprints. Success there could accelerate Down syndrome research.
Things people with down’s syndrome are tired of hearing
Picture a future where genetic therapy intervenes early, perhaps in utero or infancy, reshaping developmental trajectories.
How might that alter education systems tailored for cognitive support?
Researchers eye longitudinal studies to track edited cells over generations, ensuring stability. Preliminary data shows no major instabilities, but vigilance remains key.
In parallel, public awareness grows. Organizations like the National Down Syndrome Society advocate for inclusion, now amplified by scientific hope.
The journey from lab to clinic spans years, yet each step builds momentum. What unforeseen applications might emerge as CRISPR evolves?