Crystal Chemical Garden Experiment: Metal salts with sodium silicate turns into an Aquatic Garden
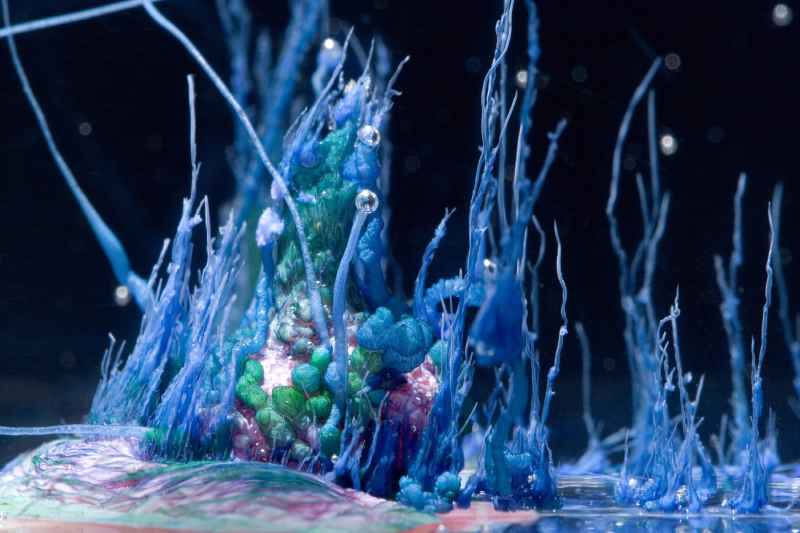
Are you ready to embark on a magical journey into the realm of chemistry and nature’s artistry? Brace yourselves, because in this article, we’ll explore the intriguing world of the “Crystal Chemical Garden Experiment” Yes, you heard that right – a garden underwater! It’s a mesmerizing fusion of science and aesthetics that will leave you spellbound.
So, grab your lab coat, put on your scientific goggles, and let’s dive deep into the world of crystal chemical gardens!
What is a Crystal Chemical Garden?
Before we plunge into the details of this aquatic marvel, let’s start with the basics.
Definition: A crystal chemical garden is a captivating and visually stunning scientific experiment that showcases the growth of intricate, delicate structures, resembling underwater gardens. These gardens are created by mixing metal salts with sodium silicate in an aqueous solution.
Now that we have a brief understanding let’s delve into the fascinating science behind it!

The Science Behind Crystal Chemical Gardens
It’s essential to comprehend the underlying chemistry that makes this experiment so enchanting. Here’s a breakdown:
1. Metal Salts
Metal salts are the primary ingredients responsible for the magical transformation. These salts, when introduced to the aqueous solution, undergo a series of chemical reactions. Each metal salt produces distinct colors and shapes, adding to the overall visual appeal.
2. Sodium Silicate
Sodium silicate, often known as “water glass,” serves as the second critical component. When sodium silicate is added to the metal salt solution, it initiates a fascinating process called osmosis.
3. Osmosis: The Magic Behind Crystal Growth
Osmosis is the key to the captivating crystal growth seen in this experiment. It’s a process where water molecules move from areas of low solute concentration to high solute concentration through a semipermeable membrane.
In this case, the membrane is a thin layer of metal silicate formed when sodium silicate comes into contact with metal ions from the salts.
As the water molecules rush in, they carry with them metal ions, forming solid metal silicate compounds. These compounds precipitate out of the solution, creating delicate, tree-like structures.

© Nevit Dilmen
Conducting the Crystal Chemical Garden Experiment
Now that we’ve demystified the science behind crystal chemical gardens, it’s time to roll up our sleeves and get practical! Here’s a step-by-step guide on how to conduct this mesmerizing experiment.
Materials You’ll Need
Before you start, make sure you have the following materials ready:
- Metal salts (choose from copper sulfate, ferrous sulfate, cobalt chloride, or nickel chloride for different colors)
- Sodium silicate solution
- A beaker or glass container
- Safety goggles
- Gloves
- Water
- A stirring rod
- Patience and a sense of wonder!
Step 1: Prepare the Aqueous Solution
- Fill your beaker or glass container with water, leaving some space at the top for the chemical reaction.
- Add a few drops of your chosen metal salt into the water. The choice of metal salt will determine the color of your crystal garden. For example, copper sulfate produces beautiful blue crystals.
- Stir the solution gently to ensure the metal salt is evenly distributed.
Step 2: Introduce Sodium Silicate
- Carefully pour the sodium silicate solution into the beaker or glass container with the metal salt solution.
- Observe the immediate reaction – you’ll notice the formation of delicate, branching structures.
Step 3: Watch the Magic Unfold
Now, the most exciting part – sit back and watch as your crystal garden comes to life! You’ll witness a gradual growth of intricate, tree-like formations. The process may take several hours, so be patient and enjoy the mesmerizing transformation.
Step 4: Document and Admire
As your crystal garden continues to flourish, take the time to document its growth through photographs or videos. The beauty of these gardens lies in their ephemeral nature, so capture the moment!

FAQs: Your Crystal Chemical Garden Questions Answered
Can I use any metal salt for this experiment?
Yes, you can use various metal salts like copper sulfate, ferrous sulfate, cobalt chloride, or nickel chloride to achieve different colors and patterns.
Is this experiment safe to conduct at home?
When conducted with proper safety precautions such as wearing goggles and gloves, and in a well-ventilated area, it can be safely done at home.
How long does it take for the crystal garden to fully form?
The time varies depending on factors like temperature and the specific metal salt used, but it typically takes several hours to a day for the garden to fully develop.
Can I reuse the materials for another crystal garden?
While you can reuse the glass container, it’s challenging to reuse the metal salts and sodium silicate solution as they undergo irreversible chemical reactions.
What happens if I mix different metal salts?
Mixing different metal salts can lead to unique and unpredictable results, producing intricate and colorful patterns.

© Bruce W. Palme
The Magic of Crystal Chemical Gardens
As your crystal chemical garden grows and flourishes, you’ll be astounded by the mesmerizing beauty it unveils.
The delicate, tree-like structures that emerge from the aqueous solution are a testament to the enchanting world of science and art colliding.
This experiment exemplifies the wonders of chemistry and the sheer beauty that can arise from the most unexpected places.
It’s a testament to the infinite possibilities of science and our ability to create beauty from the most fundamental elements.
So, whether you’re a seasoned scientist or a curious beginner, the Crystal Chemical Garden Experiment is a journey you won’t want to miss.
It’s a reminder that the world of science is not only about facts and figures but also about the sheer wonder and magic of discovery.
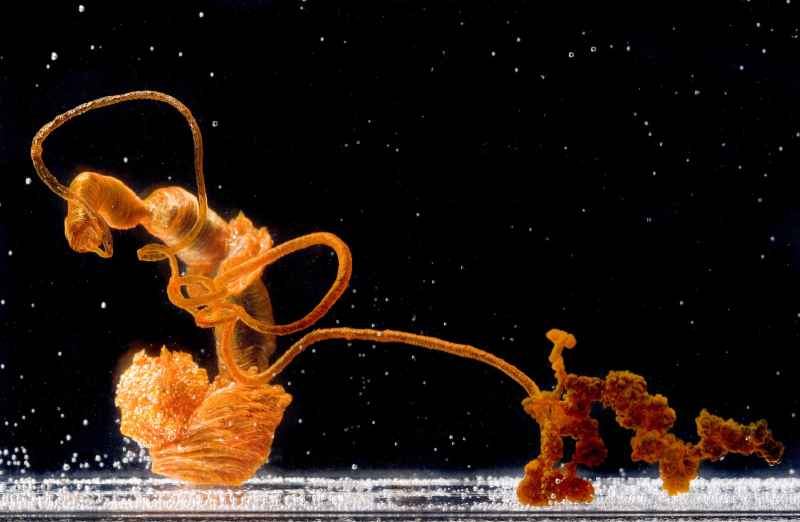
© stephanequerbes/portfolio
So, if you’re looking for an experiment that combines art and science in the most enchanting way possible, gather your materials, follow the steps, and watch in awe as your very own aquatic garden comes to life.
It’s a journey that will leave you with a newfound appreciation for the wonders of the natural world and the limitless possibilities of human curiosity and ingenuity.
Now, go forth and create your own underwater masterpiece – a crystal chemical garden that captures the essence of science and the allure of nature’s artistry!
